Which Of The Following Is A Reason Conflicts Over Zoning Decisions Can Become A Divisive Issue?, NOV 16, 2021 | City Council Afternoon Session, 56.19 MB, 40:55, 705, City of San Jose, CA, 2021-11-17T02:42:35.000000Z, 19, , , , , , 0, which-of-the-following-is-a-reason-conflicts-over-zoning-decisions-can-become-a-divisive-issue, KAMPION
For a compound to be soluble in water, it should be a polar molecule. This is because water is a. Polar molecule and polar solutes dissolve in polar solvents. The difference between the two given.
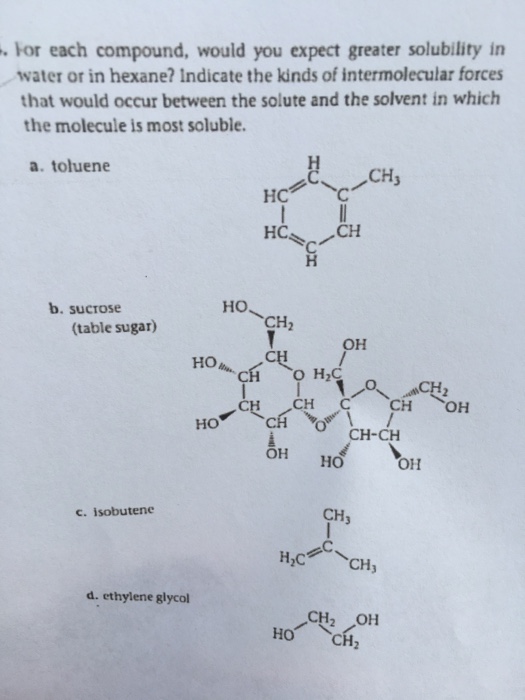
Since the oh bond is a polar bond, hoch 2 c h 2 c h 2 o h 2 o h is more. This also slightly negative, slightly negative. These are effectively neutral, all in the center. So the second molecule here, this one is going to be more soluble in water because it has more of a charge. They're going to more interactions with the different water molecules, meaning that there is going to be an increase in its scalability. Which molecules would be more soluble in water? Which molecule would you expect to be more soluble in water, ccl4 or ch2cl2? For each of the following molecules, would you expect greater solubility in water or hexane? For each case, indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble.
Solved: For Each Compound, Would You Expect Greater Solubi... | Chegg.com

Understanding the solubility rules - YouTube

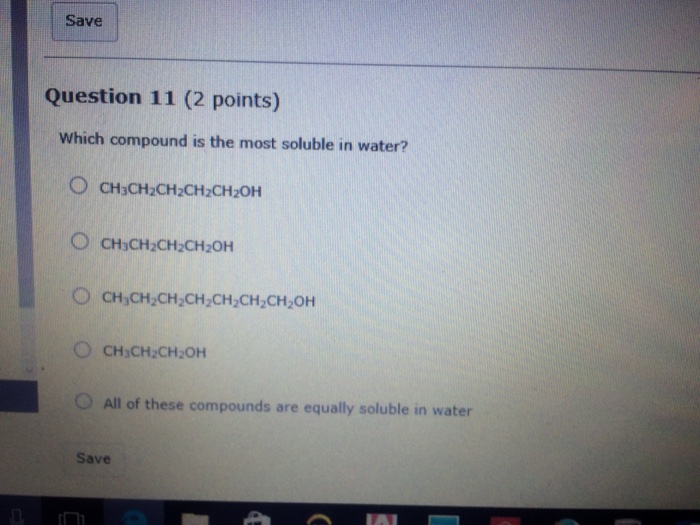
Solved: Which Compound Is The Most Soluble In Water? CH_3C... | Chegg.com
Properties water
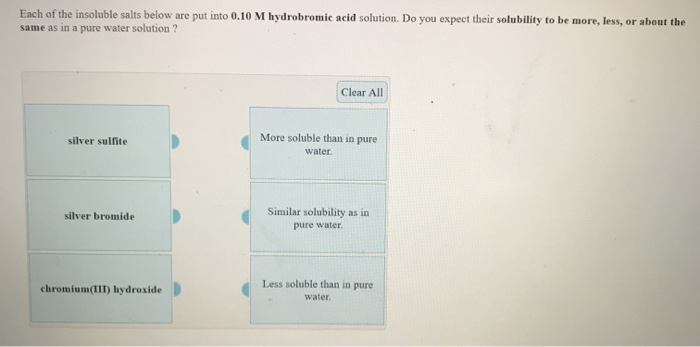
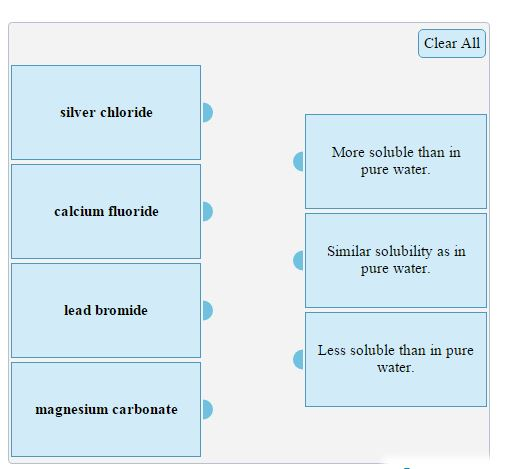
Solved: Each Of The Insoluble Salts Below Are Put Into 0.1... | Chegg.com
Please see an attachment for details | Course Hero
Solved: Each Of The Insoluble Salts Below Are Put Into 0.1... | Chegg.com
Solubility of organic compounds | Structure and bonding | Organic

B5 Deduce whether a vitamin is water or fat-soluble from its structure

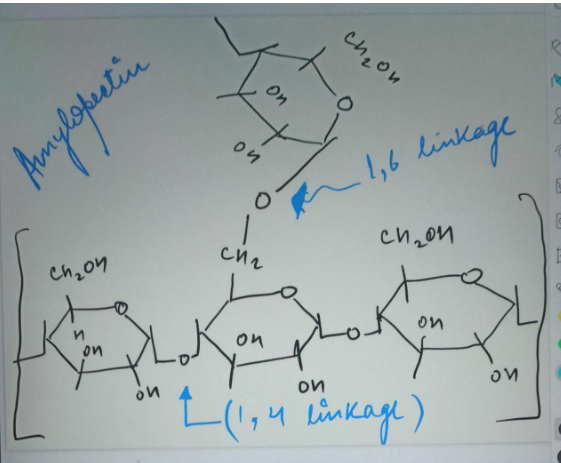
Answered: Draw the structure of starch. How does… | bartleby
Komentar
Posting Komentar