Which Of The Following Is A Reason Conflicts Over Zoning Decisions Can Become A Divisive Issue?, NOV 16, 2021 | City Council Afternoon Session, 56.19 MB, 40:55, 705, City of San Jose, CA, 2021-11-17T02:42:35.000000Z, 19, , , , , , 0, which-of-the-following-is-a-reason-conflicts-over-zoning-decisions-can-become-a-divisive-issue, KAMPION
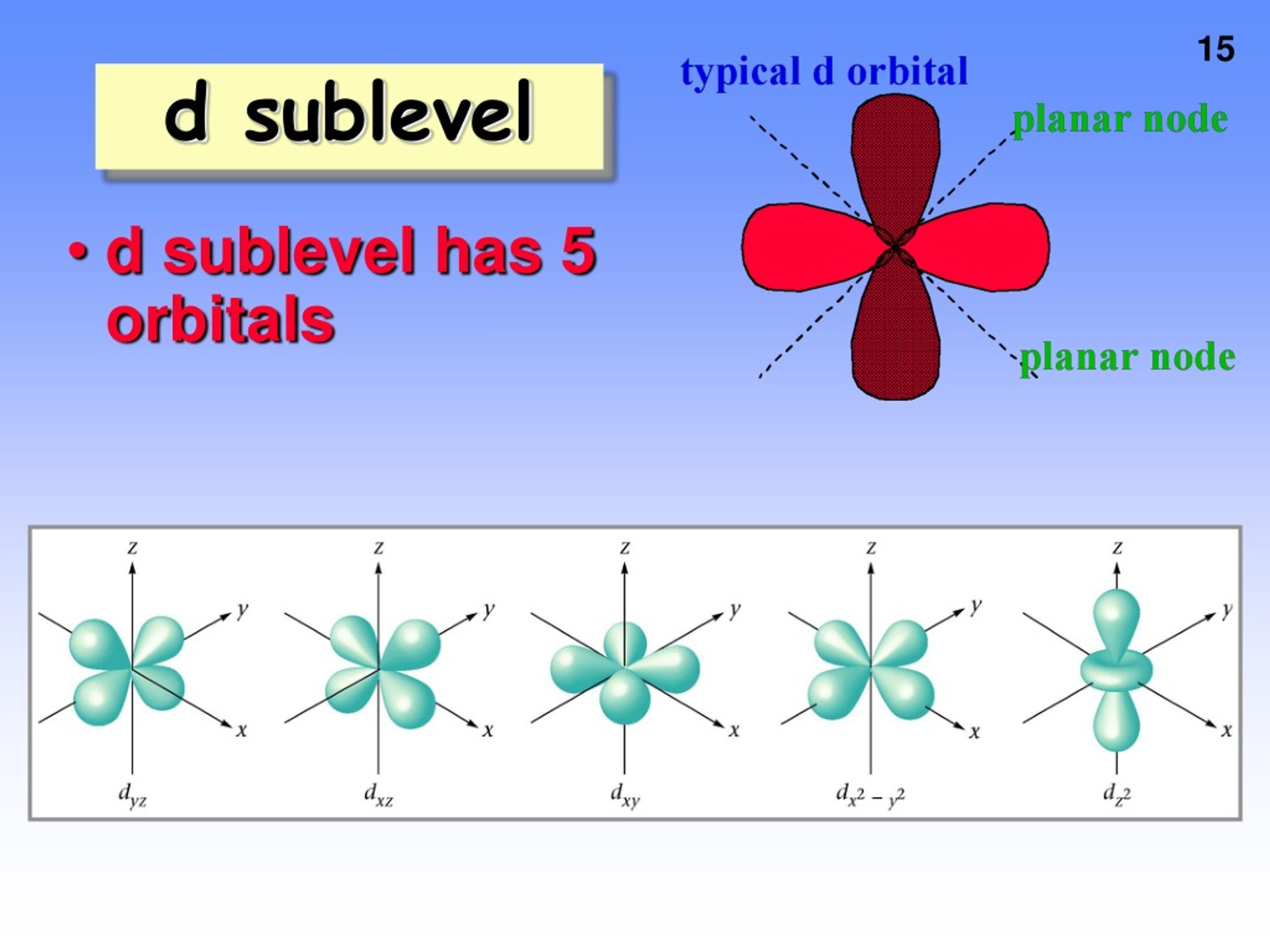
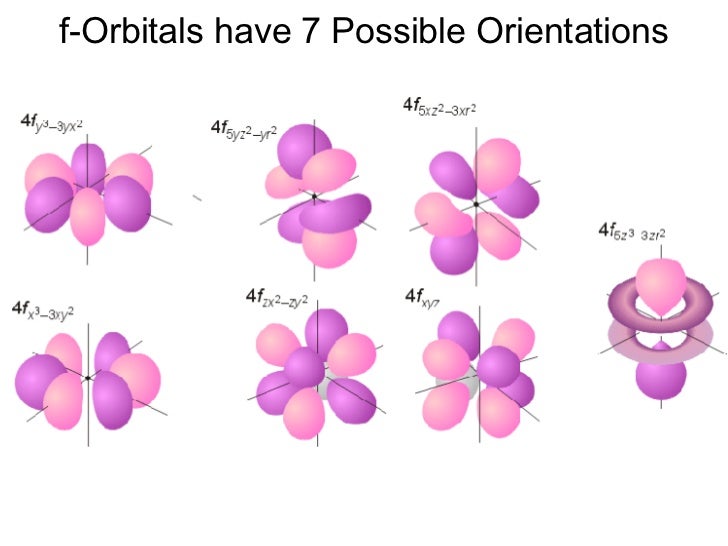
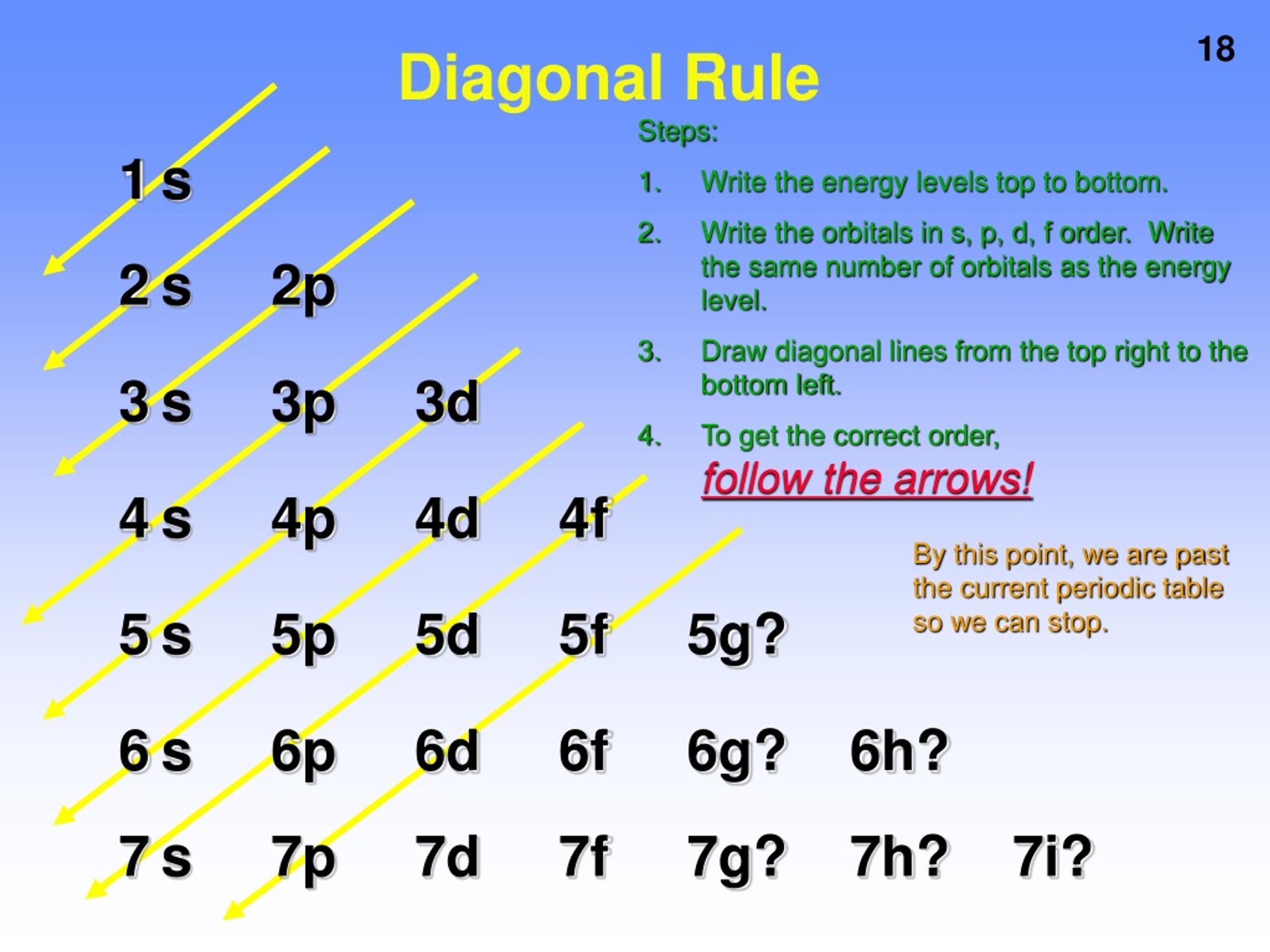
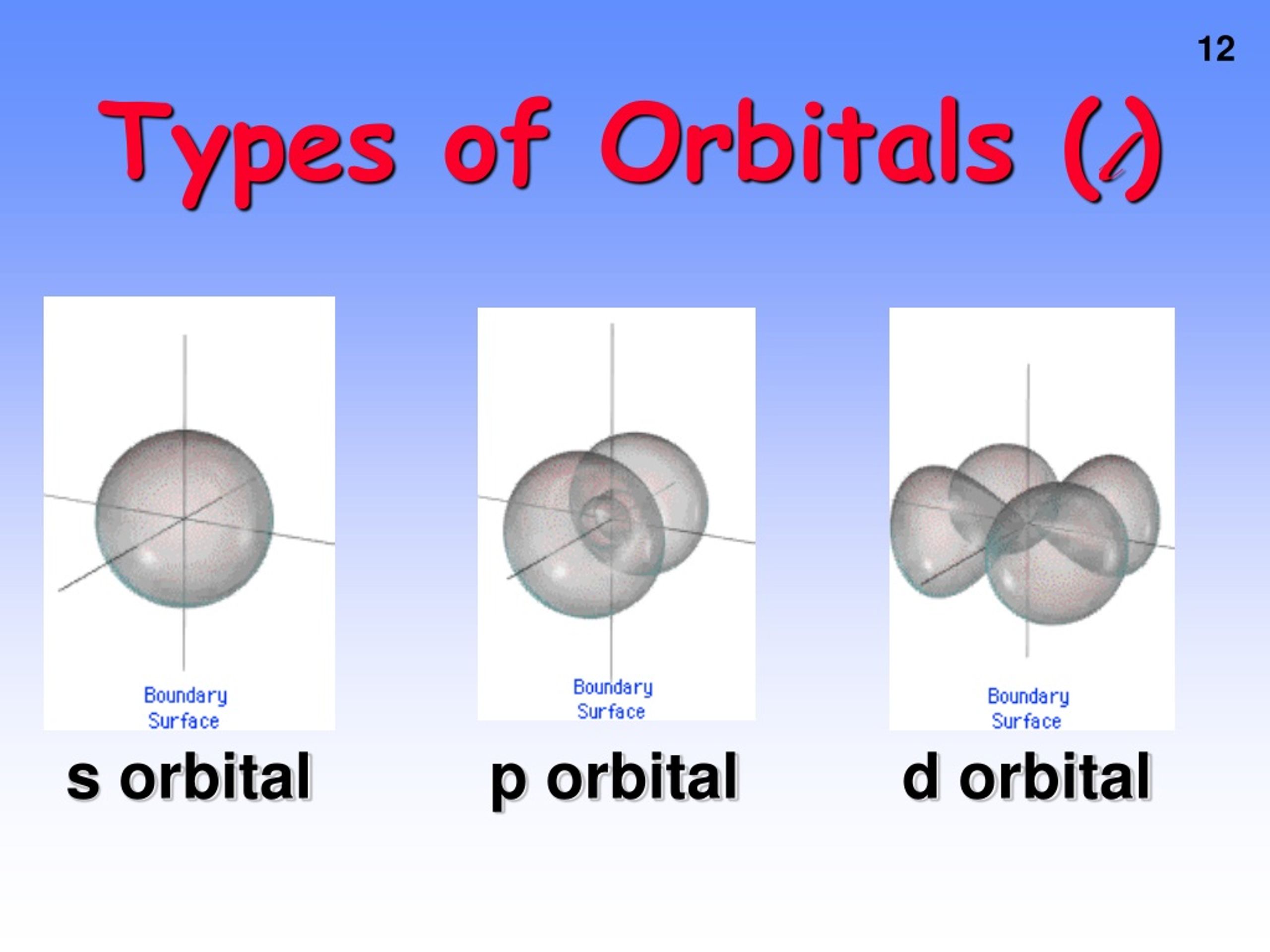
For l = 3, there are 2l + 1 = 2x3+1 = 7 ml values: So, in a given shell maximum number of f orbitals = 7. Magnetic quantum number, ml value represents the orbitals. Theoretically, there is no upper limit to the number of orbitals.
And once you go too far beyond this point,. _ what is the maximum number of f orbitals that are possible? The orbital with maximum number of possible. Orbitals are possible for the following quantum numbers? N = 4, l = 2, m =. The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons. What is the maximum number of f orbitals that are possible ? 1 3 7 5 9 determine the volume of h_2s (at 325 k and 1. 50 atm) needed to produce 50. 0 g of s. Assume that there is excess so_2 present.
PPT - Electron Configurations PowerPoint Presentation, free download

Quantum Mechanical Model of Atom - Atomic Structure - Physical

Unit3presentation
【Tips】Electron Configuration(with Video):How Do You Get the Electron

CHEMISTREE11: April 20 2011 - Electron Configuration

PPT - Electron configurations PowerPoint Presentation, free download
Unit3presentation
physical chemistry - What are the maximum number of electrons in each
PPT - Electron Configurations PowerPoint Presentation, free download
PPT - Electron Configurations PowerPoint Presentation, free download
Komentar
Posting Komentar