
Which Of The Following Is A Reason Conflicts Over Zoning Decisions Can Become A Divisive Issue?, NOV 16, 2021 | City Council Afternoon Session, 56.19 MB, 40:55, 705, City of San Jose, CA, 2021-11-17T02:42:35.000000Z, 19, , , , , , 0, which-of-the-following-is-a-reason-conflicts-over-zoning-decisions-can-become-a-divisive-issue, KAMPION
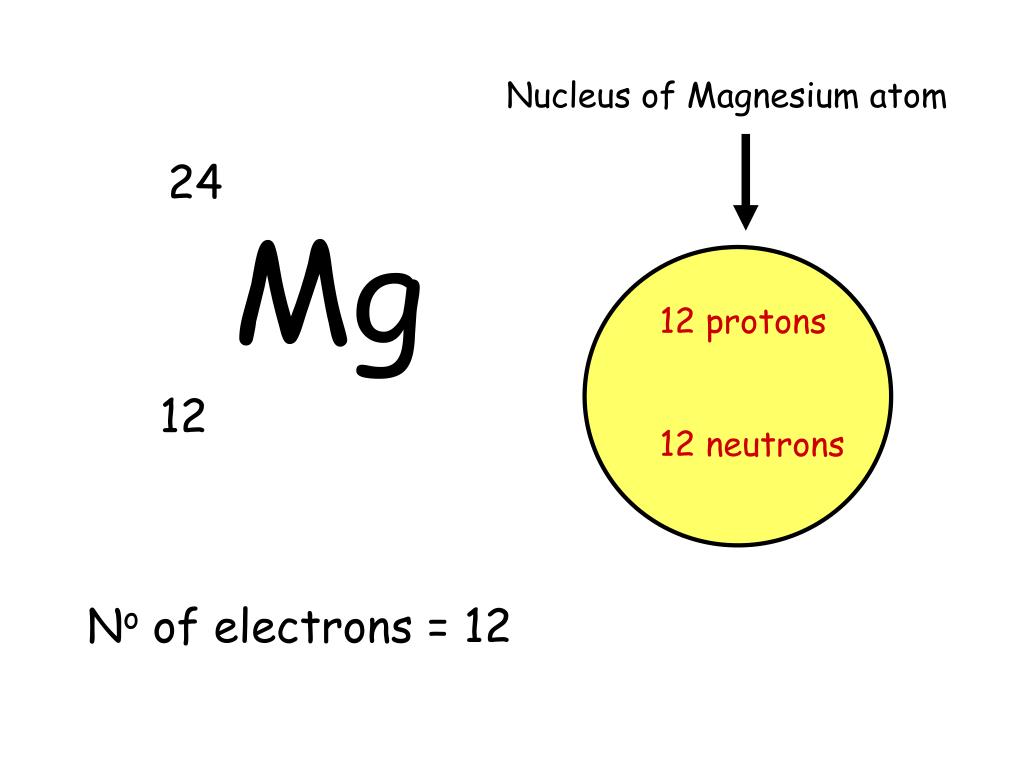
Magnesium, in its elemental form, has 12 protons and 12 electrons. The neutrons are a different matter. The neutrons are a different matter. Magesium's average atomic mass is 24. 305 atomic mass units, but no magnesium atom has exactly this mass.
A magnesium atom has the highest number of electron energy levels. The four quantum numbers are 3, 2, +2, +1, 2. The nucleus is made up of 12 protons (red) and 12 neutrons (orange). A magnesium atom has the highest number of electrons per unit. So all neutral magnesium atoms have 12 protons and 12 electrons. Isotopes of an element differ in the number of neutrons (n0). There are three stable isotopes of magnesium (mg) identified by their. 13 rowsmagnesium is the 12th element of the periodic table so its atomic number is 12. How many electrons does ca2+ have?
The magnesium atom with its protons, neutrons, and electrons is

3d Render Atom Structure Magnesium Image & Photo | Bigstock
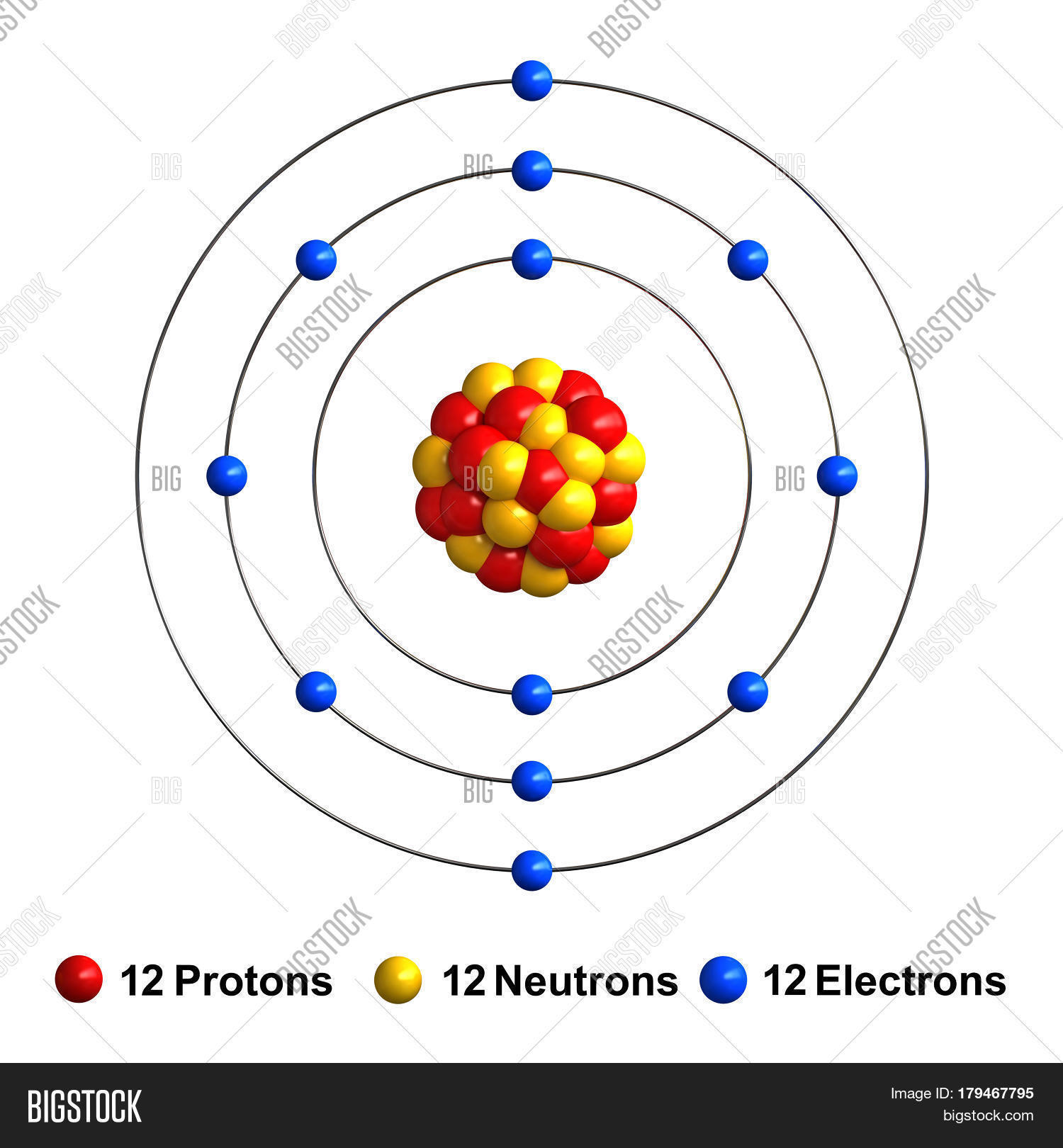
Atomic Structure: Protons, Neutrons and Electrons - Mrs. Sanborn's Site
Bohr diagrams
Properties of Magnesium Sulfate - World Update Review

Neutrons are found in the nucleus and separate the other particles so

PPT - Nuclear model of atom PowerPoint Presentation, free download - ID
Komentar
Posting Komentar